Home Page
Glycosylation Advantage
Through Cell Engineering
Driving Improved Antibody Performance Through Glyo-Precision
What is Galactosylation?
Galactosylation is the process of adding galactose (a simple sugar, or monosaccharide) to proteins or lipids, forming glycoproteins or glycolipids.
This is a type of glycosylation, a broader biological modification where carbohydrates attach to molecules.
By enhancing galactosylation of targeted treatments we can:
Optimize Antibody Function
In monoclonal antibodies (mAbs), galactosylation affects Antibody-Dependent Cellular Cytotoxicity (CDC), functions that are crucial for efficacy of cancer and autoimmune therapies.
Enhance Drug Stability
Enhanced galactosylation helps prevent protein degradation, improving the drug’s shelf life and effectiveness.
Reduce Immunogenicity
Suboptimal glycosylation (like under-galactosylation) can trigger unwanted immune responses, impairing drug tolerability.
Control Clinical Profile - Pharmacokinetics & Pharmacodynamics
Level of galactosylation affects how long a drug stays in the bloodstream and how well it binds to target cells. Optimised galactysolation improves pharmacologic profile.
In monoclonal antibodies (mAbs), galactosylation affects Antibody-Dependent Cellular Cytotoxicity (CDC), functions that are crucial for efficacy of cancer and autoimmune therapies.
Problem
- Galactosylation is a critical glycosylation event during biologics manufacturing.
- Variable galactosylation leads to significant antibody development challenges and potential failure.
- Inconsistent pharmacology
- Compromised safety/efficacy product profile
- Scrutiny from regulatory authorities
Solution
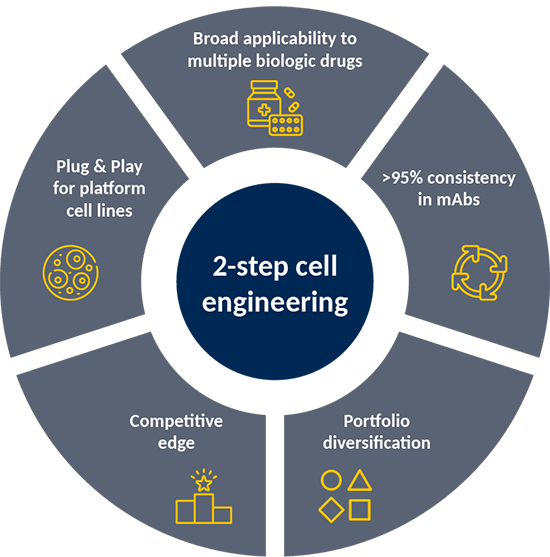
- Glyvantis Bio’s novel 2-step cell engineering technology is set to become a new reference standard in biological manufacturing processes.
- Maximizing galactosylation
- Reproducible with high level of consistency
The Glyvantis Bio Solution
is a unique, proven and reproducible cell engineering technology to optimise galactosylation for antibodies to;
- Optimize function
- Enhance stability
- Reduce immunogenicity
- Control clinical (PK/PD) profile
And additionally reduce the galactosylation variability.
Consequences of Variable Glycosylation
There are a series of consequences of variable glycosylation amongst some of the most important biologics:
Antibody function
- CDC activity influenced by glycosylation of Fc region, with regulatory scrutiny of clinical underperformance (rituximab, other CD20 mAbs, infliximab).
- Variation affecting Fc effector function includes ADCC drift with trastuzumab.
- Impact on potency in VEGF inhibition (bevacizumab).
Pharmacology
- Tenecteplase clearance rate dependent on extensive glycosylation, biosimilar delays due to challenges in matching pattern.
- Glycoform heterogeneity significantly impacts EPO’s serum half-life and activity.
- Biosimilars face challenges matching glycosylation pattern of anti CD20s, that influences half-life, clearance and distribution.
Immunogenicity
- Immunogenic glycans such as α-Gal can produce severe allergic reactions (cetuximab).
- Immune-related reactions with bevacizumab, influenced by glycosylation pattern.
Tolerability
- Multiple monoclonal antibodies with narrow therapeutic index and sensitivity to glycoslyation variation e.g., gemtuzumab, alemtuzumab.
- Variable PK can result in excessive on-target toxicities e.g., hypertension with bevacizumab, skin rash with cetuximab.
